by Alex | Mar 7, 2016 | healthcare, individual health insurance, Pharmaceutical Industry
Brand Name Drugs Are a Waste of Money?
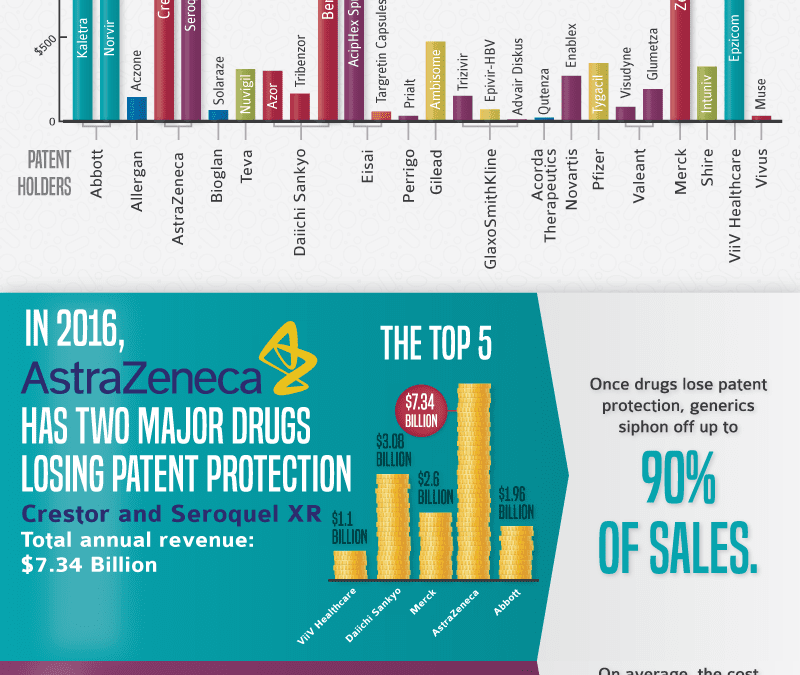
Generics or Brands? This is not a new question and has been discussed. Our blog post “Generic Drugs vs. Brand Name – Are there any differences?” was covered in 2009. But since last decade most brand drug’s patents have fallen of a cliff. See popular Rx expirations below and infographic of “Major Drugs Going Off-patent in 2016.”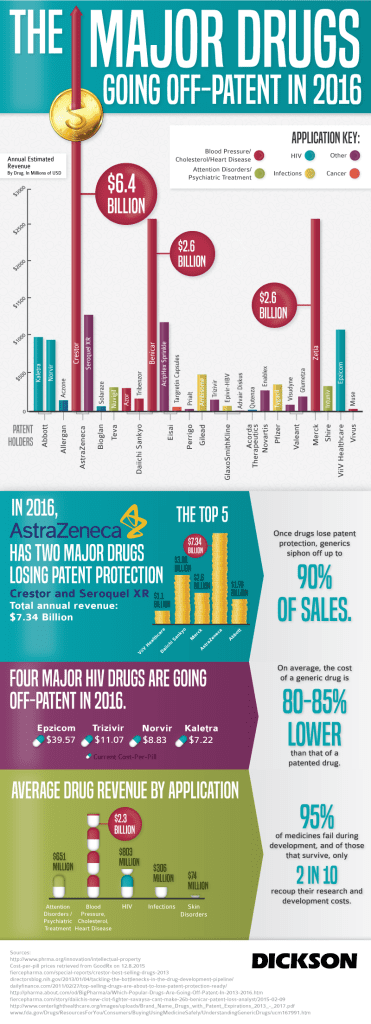
- Lipitor
- Seroquel
- Crestor
- Prevacid
- Nexium
- Zyprexa,
- Plavix
- Singulair
- Lexapro
- Enbrel
According to Vox article “Stop wasting money on brand-name drugs “On average, generics cost 80 to 85 percent less than name-brand medicines, according to the Food and Drug Administration (FDA). And buying unbranded drugs isn’t like opting for cheap toilet paper or no-name face cream: Less expensive here doesn’t necessarily mean lower quality.”
Many people have heard that switching to a generic medication will save them money. One of the questions we hear most often is, “How do generic medications compare to their brand name counterparts?” Knowing the facts about generics versus brand names can help make us all better consumers.
What’s the difference between generic and brand-name drugs?
Generic medicines are chemically equivalent to the original brand-name drugs and work just as well for nearly all patients. Many people are concerned that because generic drugs are often much cheaper than the brand-name versions, the quality and effectiveness have been compromised to make a less expensive product. The FDA requires that generic drugs be as safe and effective as the original brand name drugs. Generic drugs are copies of brand name drugs that have exactly the same dosage, intended use, effects, side effects, route
of administration, risks, safety, and strength as the original drug. In other words, the pharmacological effects of generic medications are exactly the same as those of their brand name counterparts. It is important, however, to check with a Physician and have a conversation about alternatives since generic drugs may contain slightly different INACTIVE ingredients. These are things like binding materials, dyes, preservatives, and flavors.”
Another common myth is that generic drugs take longer to work. The FDA requires that generic drugs work as fast and as effectively as the original brand name products.
When a drug loses patent protection, often only one generic version is on sale for the first six months, so the price falls a little bit initially. Then, several other generic makers typically jump in, driving prices down dramatically.
What are the actual costs differences?
Last year, the average generic prescription cost $72, versus $198 for the average brand-name drug, according to consulting firm Wolters Kluwer Pharma Solutions. Those figures average all prescriptions, from short-term to 90-day ones.
Average copayments last year were $6 for generics, compared with $24 for brand-name drugs given preferred status by an insurer and $35 for non-preferred brands, according to IMS Health. Protonix, for severe heartburn, now costs just $16 a month for the generic, versus about $170 for the brand name. And of the top sellers that soon will have competition, Lipitor retails for about $150 a month, Plavix costs almost $200 a month and blood pressure drug Diovan costs about $125 a month. For those with drug coverage, their out-of-pocket costs for each of those drugs could drop below $10 a month.
All patients are encouraged to discuss generic alternatives with their physicians prior to filling a prescription. The doctor and the patient should agree on the best course of treatment for any diagnosed medical condition.
Resource:
Discount Rx Card: www.bonusbenefit.com
Pharmacy and Rx Costs Look Up: http://www.rxpricequotes.com

by admin | Aug 17, 2009 | COBRA, Dependent Coverage, family health insurance, group health insurance, latest health insurance news, Pharmaceutical Industry, regional health insurance co-ops

Health Care Reform!
Ok so unless you’ve been stuck in the Arctic for a year you’ve been hearing a lot about this heated topic. Everyone has strong feelings about it evidently, I myself included, but I have stayed away from the fray for the most part.
As congress takes their August recess and those who still have jobs are on vacation I thought its a good time to put my two cents into it.
This well done score card brought to you by Empire Blue Cross is a great illustration of the leading proposals and voices in Washington. A nicely published overview by the Lewin Group is actually a great read on the proposed Government Sponsored Health Plan. The analysis covers the bill as it appeared on July 15, 2009.
Bills Key Provisions:
- Require all Americans to purchase health insurance or be fined, although those making less than $88,000 annually would be able to get a subsidy.
- Get rid of copays and deductibles for preventative care
- Make it illegal to deny coverage for pre-existing conditions
- Create a public plan
- Raise taxes for the wealthy – as much at 5.4 percent for incomes above $350,000
But what are we really talking about? A Government Plan to compete with private payers? Really?
The assumption in the study is that the government plan pays Medicare Rates. Provider reimbursements are on average 70% of private insurance reimbursements. The specter of physicians opting out of this plan is rather daunting as they would be giving up the single largest payer.
How does a private insurer compete with a government plan? Imagine a Government-owned subsidized Automobile competing with private companies? Would they not print more tax payer money and pump them up? Oh wait that’s already happened in Detroit, bad example.
The President claims that a government plan does already work and its name is Medicare. Yet, Medicare we are also told will go broke as early as by 2018 reported by Washington Post. Medicare, meanwhile, now pays private insurers to take care of seniors under the Medicare Advantage Plans. It is cheaper for the government to do this than to manage it themselves
As brokers, we work with the AARP Oxford Secure Horizons Program where some plans are $0 premium and include a fairly sizable network.
So which one is it? Does the Medicare plan work now and is proof of what’s to come or is it costly and inefficient and unsustainable?
Clearly the costs are indeed high and I question what insurers are thinking with some of the rate increases. This year, especially, I’ve seen increases of over 20% from the top carriers.
Speaking of Medicare, the Part D Plan in 2003 was just a gift to the Pharmaceutical industry’s under the Bush administration. Many people didn’t realize that the language used barred the U.S. from negotiating drug pricing. How can Canada with an entire population of 33 mill pay 50% on the dollar while 40 million US seniors pay full retail? Coincidentally, the legislators of Medicare Part D earned themselves nice cushy paying Pharmaceutical jobs within 1 year.
Obama has easily gotten concessions from Big Pharma, Insurers and the AMA (provided there is tort reform) already and I applaud him for it. There probably is even more good news to come on this.
What may be an interesting possible outcome are Regional Health Insurance Co-ops. These are a bridge between government and non-government options. The co-op alternative, led by Sen. Kent Conrad (D-ND), continued to gain traction on both sides of the aisle. The plan would call for the creation of nonprofit health cooperatives in lieu of public health insurance options. Said Sen. Baucus, “.The Conrad approach has got legs…it’s quite viable.”
On the House side, Rep. Roy Blunt (R-MO), chairing the Health Care Solutions Group, released an alternative to the House Democratic plan that he “hopes will receive bipartisan support.”
An example of this is GroupHealth in Washington State. “At Group Health, doctors are rewarded for consulting by telephone and secure e-mail, which allows for longer appointments. Patients are assigned a team of primary care practitioners who are responsible for their well-being. Medical practices, and insurance coverage decisions, are driven by the company’s own research into which drugs and procedures are most effective.” A good piece in last months’ NYT. discusses this.
There are many versions of this and perhaps there ought to be Federal provisions and overall guidelines but with regional flexibility afforded to each state. This topic requires further discussion and I will tackle it next month.
Enjoy the rest of your summer!!!

by admin | Jan 16, 2009 | healthcare, Pharmaceutical Industry
 GENERIC VERSUS BRAND NAME MEDICATIONS
GENERIC VERSUS BRAND NAME MEDICATIONS
Many people have heard that switching to a generic medication will save them money. One of the questions we hear most often is, “How do generic medications compare to their brand name counterparts?” Knowing the facts about generics versus brand names can help make us all better consumers.
All generic drugs are reviewed and approved by the United States Food and Drug Administration (FDA) or in other countries, by an equivalent federal regulatory body. The regulatory boards all require that generic drugs have the same active ingredients, quality, strength, purity, and stability as brand name drugs. They also must have the same dosage form, whether you swallow it (pill/tablet/capsule/caplet), drink it (liquid), or inject the medication.
Many people are concerned that because generic drugs are often much cheaper than the brand-name versions, the quality and effectiveness have been compromised to make a less expensive product. The FDA requires that generic drugs be as safe and effective as the original brand name drugs. Generic drugs are copies of brand name drugs that have exactly the same dosage, intended use, effects, side effects, route of administration, risks, safety, and strength as the original drug. In other words, the pharmacological effects of generic medications are exactly the same as those of their brand name counterparts. Another common myth is that generic drugs take longer to work. The FDA requires that generic drugs work as fast and as effectively as the original brand name products.
Generic drugs are cheaper because the manufacturers do not have the expense of developing and marketing a new drug. When a company brings a new drug to the market, the firm has already spent substantial money on research, development, marketing and promotion of the drug. A patent is granted which gives the company that developed the drug exclusive rights to sell the drug for as long as the patent remains in effect.
As a patent nears expiration, manufacturers may apply to the FDA for permission to make and sell generic versions of a drug. Since there are no startup costs for development of a new drug, other companies can afford to make and sell it for a less expensive amount. When multiple companies produce and sell a drug, the competition drives the price down even further.
The FDA applies the same standards for all drug-manufacturing facilities. Many companies manufacture both brand name and generic drugs. In fact, the FDA estimates that 50% of the generic drugs are produced by the same company that created the initial brand name drug.
Generic versions of drugs have different colors, flavors, or combinations of inactive ingredients than the original medications because United States trademark laws do not allow the generic drugs to look exactly like the brand name medication. However, the active ingredients must be the same in both generic and brand name medications, ensuring that both have the same effectiveness in treating a medical condition.
A physician’s decision to prescribe a brand name over a generic is based on the needs of the patient. Three in four physicians allow generic substitutes for brand-name drugs, even though most of them do not have a “dispense as generic” box on their prescription pad.
Pennsylvania law allows pharmacists to substitute generic drugs for original brand named drugs, unless the person writing the prescription, or the patient, directs otherwise. If the physician writes “Brand Necessary” or “Dispense As Written (DAW)” on the prescription, the pharmacist may not substitute the brand name medication with a generic alternative.
All patients are encouraged to discuss generic alternatives with their physicians prior to filling a prescription. The doctor and the patient should agree on the best course of treatment for any diagnosed medical condition.
A Guide to Legal Issues in Health Care: Prescription Drugs Retrived September 16, 2006 from http://www.upenn.edu/ogc/legal/pred.html Are Generic Medications the Same as Branded Counterparts? Retrieved September 16, 2006 from http://counsellingresource.com/medications/discount-drugs/generics.html COMMONWEALTH OF PENNSYLVANIA GENERIC DRUG EQUIVALENCY/SUBSTITUTION LAWS & REGULATIONS Retrieved September 16, 2006 from http://ecapps.health.state.pa.us/pdf/ddc/generic33.ps.pdf#search=%22pennsylvania%20drug%20dispensing%22How Physicians Feel About Prescribing Generics Retrieved September 16, 2006 from http://www.aarp.org/health/affordable_drugs/physiciansandgenericdrugs.html
Prescription Drugs – Generic vs Brand Name Retrieved September 16, 2006 from http://www.crossborderpharmacy.com/Canadian-Generics-vs-Brand-Name.htmlStoppler, MD, M., Generic Drugs, Are They As Good as Brand-Names? Retrieved September 16, 2006 from http://www.medicinenet.com/script/main/art.asp?articlekey=46204




